Objective
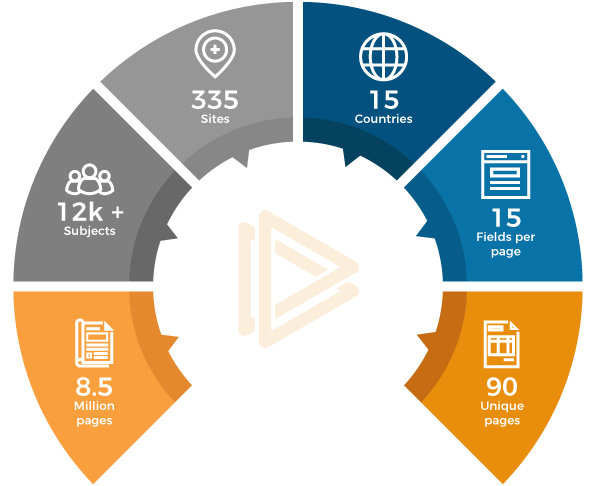
A leading Fortune 500 pharma multinational in US was looking for an EDC solution for their late phase Immunology and infectious disease TA. Since the quantum of trial Parameter was enormous, the client wanted to have a platform which can be scalable quickly as the trial progresses.
Benefits
Acceliant helped by:-
- Creating multiple version of CRF.
- System Performance – 250 real time concurrent user access the system without any lag.
- Easy and configurable query work flow – As the trial as huge Adverse events.