It’s a dangerous world we live in. One which is fraught with the dangers of sudden affliction to a host of physical and mental diseases. Does this mean a desolate, hopeless future? Not if we simultaneously measure the exponential advances medical science has made over time. Not if we evaluate how the quality of medical services has improved by leaps and bounds. Not if we account for the state-of-the-art drugs and medical devices that have been engineered. And finally, not if we quantify the number of lives this progress is helping save on a daily basis.That said, the decrease in the mortality count – thanks to advanced technology – has brought an increase in information with itself. A monumental rise in information about patients. Every single patient. Medical reports which exhaustively list the symptoms, diagnoses, procedures and medication for every ailment a single patient may have suffered – and may / may not have recovered – from. But medical care comes at a cost. Sometimes a high one. So what does a person who can’t afford all the involved expenses do? Simple – he enrols for insurance. And furnishes all this data to his insurance company to make a suitable medical claim. Irrespective of the nature and severity of the affliction.Clearly, this vast data quantity needs to be represented in a concise format. Enter the Medical Coder. Medical coders translate this information into a set of codes, which make up an important part of a medical claim.Let’s walk you through some statistics before explaining and why medical coding is so important in the present context.According to the Centers for Disease Control (CDC), there were over 1.2 billion patient visits in the past year. This figure includes visits to physicians’ offices, hospital outpatient facilities and emergency rooms. Let’s assume there were only five pieces of coded information per visit. This would translate into 6 billion individual pieces of information that need to be transferred annually. Suffice to say that in a system burdened with data, medical coding enables the efficient and effective transfer of vast amounts of information
The Acceliant advantage
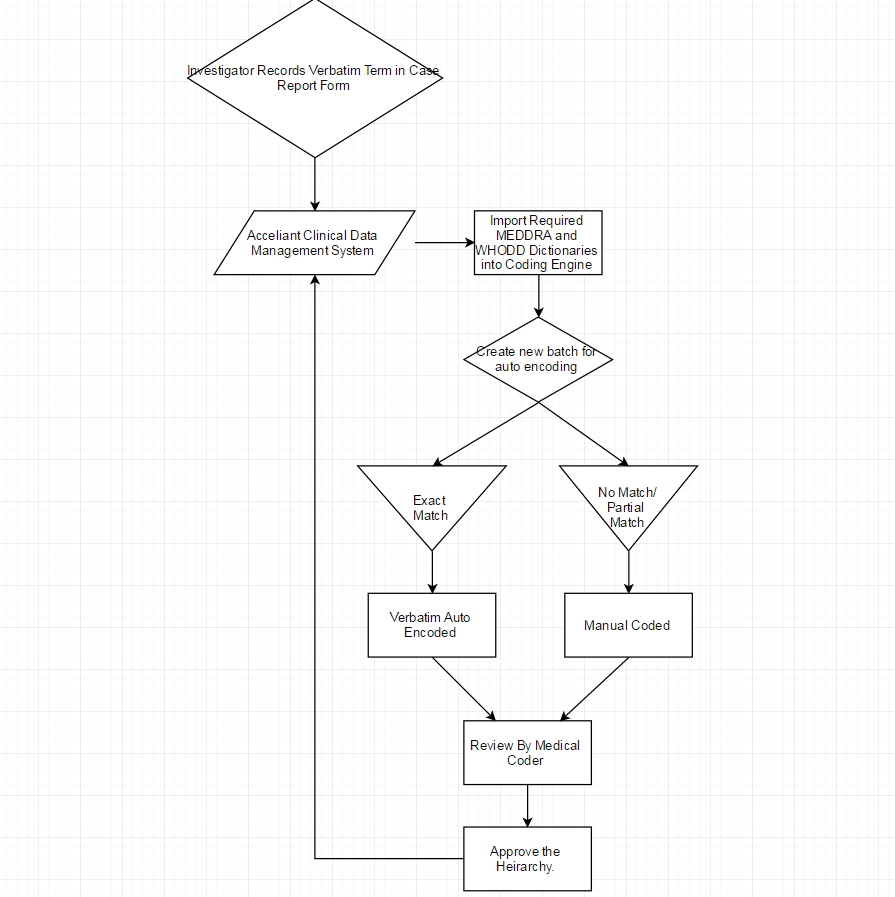
Medical coding can be both manual and automated, i.e. medical terms which fail to get auto encoded have to coded manually after referring to relevant dictionaries. The question is what if both could be done in tandem in an integrated fashion? Why not?! Acceliant provides precisely this sort of integrated coding mechanism, allowing users to code terms using the Medical Dictionary for Regulatory Activities (MedDRA) and/or the WHO Drug Dictionary Enhanced (WHODrug) dictionaries. Acceliant’s coding engine not only furnishes highly accurate auto encoded terms, it also allows users the flexibility to search these dictionaries for more accurate results as well as set threshold limits in auto encoding terms. Searches can be performed using multilevel hierarchy options for both MedDRA and WHODrug.Coding is ideally performed on validated and cleaned data by users for review and discrepancy management. The terms can be coded in batches and can be auto encoded, by a process known as "auto encoding", the terms which fail to get "auto-encoded" have to be coded manually by lookup through the dictionaries.Auto Coding is a process in which the term recorded in the CRF gets coded automatically if it matches with the appropriate term available in the dictionary.
Manual coding is a process in which the term recorded in the CRF may not get auto-encoded as the recorded term does not match the appropriate hierarchy in the available dictionary, and requires to be manually coded by the user by lookup in the appropriate dictionary.It does not mean that all the verbatim terms that get entered in the CRF get coded, for unclear terms, the user can raise a query requesting more information in-order to select the exact term from the dictionary.In some cases, the entered verbatim term may be a something which requires no coding, in this case, the user has an option to set the verbatim to DNC (Do Not Code Option).
Acceliant additionally provides options to search the MedDRA and WHODrug dictionaries in multiple hierarchies to get the correct match, apart from suggesting multiple options to users about picking the correct match.For studies which run for longer durations, Acceliant supports multiple versions of these dictionaries to facilitate the movement of encoded terms to later versions of both/ either dictionaries.
The below mentioned flowchart illustrates this process.
Medical Dictionary for Regulatory Activities (MedDRA)
Medical Dictionary for Regulatory Activities (MedDRA®) is a medical coding dictionary developed by the Maintenance and Support Services Organisation (MSSO). MedDRA® is supported by the International Conference on Harmonisation (ICH) on Technical Requirements for Registration of Pharmaceuticals for Human Use. Interestingly, prior to the development of MedDRA, an internationally accepted medical terminology repository for biopharmaceutical regulatory purposes did not exist.
MEDDRA is used for coding:
- Medical terms generated during all clinical trial phases, excluding animal toxicology
- Therapeutic indications including signs, symptoms, diseases, diagnosis, or prophylaxis of disease, and modification of functions
- Coding names and quantitative results of investigations, surgical procedures and medical/social/family history
MedDRA releases 2 versions in a year – one in March and the second in September. One can obtain access to the MedDRA terminology annually, by renewable subscription. Each subscription brings all MedDRA updates that incorporate approved changes and additions
MedDRA has five hierarchical levels as listed below
- Low Level Term (LLT)
- Preferred Tem (PT)
- High Level Term (HLT)
- High Level Group Tem (HLGT)
- System Organ Class (SOC)
Low Level Term (LLT) is the lowest level of the terminology. Each LLT is linked to only ONE PT. A PT distinctly describes a symptom, sign, disease, diagnosis, therapeutic indication, investigation, surgical, or medical procedure and medical, social, or family history characteristics.1
High Level Term (HLT) is a superordinate descriptor for PTs linked to it. High Level Group Term (HLGT) is a superordinate descriptor for one or more HLTs related by anatomy, pathology, physiology, etiology, or function. System Organ class (SOC) is the highest level of hierarchy. The SOCs are grouped by etiology, manifestation site and purpose.
WHO Drug Dictionary Enhanced (WHO-DD Enhanced)
The World Health Organisation Drug Dictionary (WHODrug) is maintained and updated by the Uppsala Monitoring Centre (UMC). It is arguably the most comprehensive dictionary on medicinal product information. It is used by several drug regulatory authorities, as well as various pharmaceutical companies and contract research organizations (CROs). As of today, there are three variants of this dictionary that are in use.
The WHO Drug Dictionaries contain information about the Medicinal Products. This information is used to identify a term (medicinal product) closely matching with the term reported on DCI.
ATC classification is integral part of the dictionary. This is used to classify the medicinal product to the main therapeutic use of the active ingredient/s
- LEVEL 1: Anatomical main group
- LEVEL 2: Therapeutic subgroup
- LEVEL 3: Pharmacological subgroups
- LEVEL 4: Chemical subgroups
- LEVEL 5: Chemical substance
The WHO Herbal Dictionary contains almost all herbal entries that have been entered into WHO Drug Dictionary over the years. From 2005 all herbals will be included exclusively in the WHO Herbal Dictionary. The WHO Herbal Dictionary is classified with the Herbal Anatomical Therapeutic Chemical (HATC) classification.The WHO Drug Dictionaries contain information about the Medicinal Products. This information is used to identify a term (medicinal product) closely matching with the term reported.
